
Introduction:
Determining the origin of water (H2O) and chemically bound hydroxyl radicals (-OH) on airless bodies is necessary and critical for understanding the origin, evolution, and overall accessibility of this potential resource for future human exploration in our Solar System. Chemically bound hydroxyls are formed within the lunar regolith when the solar wind bombards the surface. A small fraction of these bound hydroxyls will ultimately react resulting in the formation of molecular water in a process known as recombinative desorption. This process involves the thermally activated reaction of two hydroxyl groups near one another which culminate in the release of molecular water.
Description:
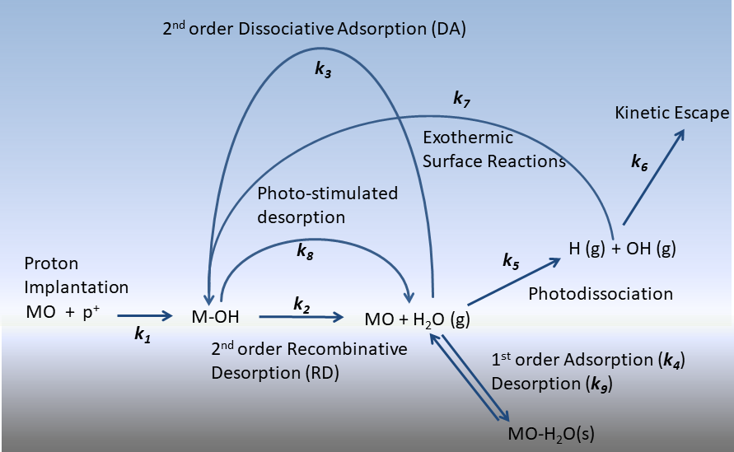
This figure below depicts the overall chemical kinetic model utilized to simulate the evolution of hydroxyls formed via solar wind irradiation on the lunar surface. Briefly, chemically bound hydroxyls (-OH) are formed via proton implantation from the solar wind and then removed from the lunar soil via recombinative desorption or photo-stimulated desorption ultimately producing gas phase molecular water. From here, the gas phase water has several different reaction pathways including dissociative adsorption, physisorption, and photo-dissociation. For this paper, we measured the recombinative desorption rate of an actual lunar regolith (Apollo sample 10084) through a technique known as temperature program desorption. Results of this numerical simulation incorporating the measured recombinative desorption rate are shown in the figure below. As the rate k2 depends on coverage (i.e., lower coverage slower rate and vice versa), the bound hydroxyls formed through proton implantation will ultimately reach a critical level where the rate of loss is balanced with the rate of formation, resulting in a very slight diurnal variation over the lunar day. Over time, the hydroxyl signal will appear stronger in the polar regions due to the lower temperatures and thus slower loss rates.
Summary:
The latitude dependence of bound hydroxyls in the regolith results from formation and loss pathways induced by the solar wind production and subsequent recombinative desorption. This mechanism forms gas-phase molecular water on the sun-lit side, which further undergoes photo-dissociation and dissociative adsorption. The cycle results in a latitude-dependent concentration of OH groups as depicted in the video below.

Take home message:
So, the next time you are looking at a full moon, realize that a tiny amount of water is undergoing a very dynamic experience of creation and destruction right before your eyes!
For more information the reader is encourage to read the full paper, “Jones, et. Al. Geophysical Research Letters, Vol 45, pg 10959-10967, 2018”

