
The idea that water exists on the Moon has been around for many years, and its presence would provide a useful resource for human exploration. When the solar wind bombards the lunar surface, it can cause a reaction that creates molecular water. This paper describes the process and how it was modeled in the laboratory.
Excerpt from the Paper:
Determining the origin of water (H2O) and chemically bound hydroxyl radicals (-OH) on airless bodies is necessary and critical for understanding the origin, evolution, and overall accessibility of this potential resource for future human exploration in our Solar System. Previous studies of terrestrial minerals bombarded with protons mimicking solar wind have resulted in the appearance of a mid-infrared (2.8 µm) absorption associated with chemically bound hydroxyls suggesting that this observed feature on the moon originates from the solar wind. In addition our group has demonstrated that molecular water can be produced from minerals with saturated hydroxyl surface sites at lunar relevant temperatures in a process known as recombinative desorption. This process involves the thermally activated reaction of two hydroxyl groups near one another and release molecular water.
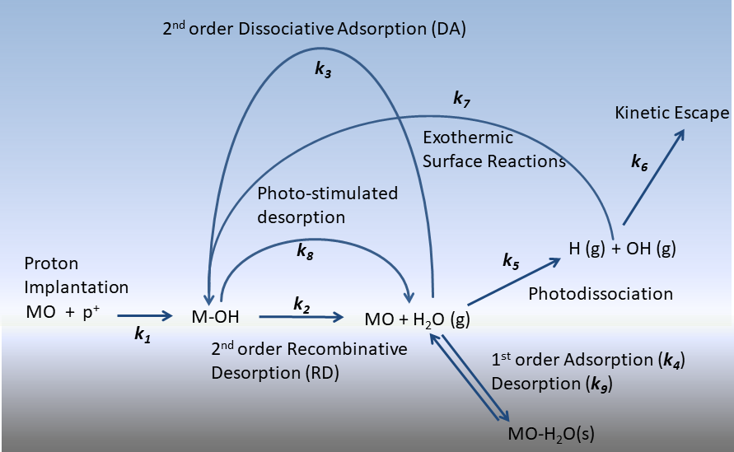
This figure depicts the chemical kinetic model utilized to model the evolution of hydroxyls formed via solar wind irradiation on the lunar surface. Briefly, chemically bound hydroxyls are formed and then removed via recombinative desorption or photostimulated desorption ultimately producing gas phase water. From here, gas phase water has several different reaction pathways including dissociative adsorption, chemisorption, photodissiciation. Over time, the hydroxyl signal will appear stronger in the polar regions due to the lower temperatures. A slight diurnal change is observed in the equatorial regions producing a small amount of molecular water each lunation.
Utilizing available data sets on non-thermal and thermal rates of water formation and desorption from various hydroxyl-terminated metal oxides (SiO2, TiO2, and Al2O3), and experimental temperature program desorption data of Apollo sample (10084) lunar mare regolith, the 2.8 µm optical signature on the Moon is modeled. Specifically, this band results from formation and loss pathways induced by the solar wind production of chemically bound hydroxyls and subsequent recombinative desorption. This mechanism forms gas-phase H2O on the sun-lit side, which further undergoes photo-dissociation and dissociative adsorption. In conjunction with the continuous implantation via solar wind, re-hydroxylation of the surface is persistent, described schematically below. The cycle results in a latitude-dependent concentration of OH groups with a slight diurnal behavior as depicted in the video.
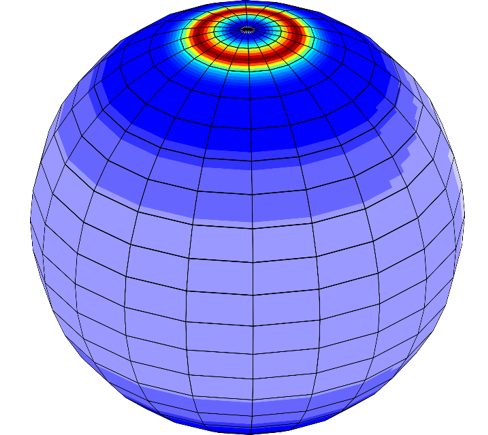

On the left is the hydroxyl concentration on the lunar surface after modeling the chemical kinetic pathways described in Figure 1 for 3600 Lunar Days. On the right is a video showing the progression of hydroxyl concentration. Early on, the peak concentrations are near the equator as expected if the solar wind implantation has no loss channels. After time, peak concentrations for the colder latitudes build up resulting in concentrations that match observations. For more information the reader is encourage to read the full paper, “Jones, et. Al. Geophysical Research Letters, Vol X, pg Y, 2018”

